

Salt Batteries: A Promising Alternative to Lithium-Ion Batteries
Salt batteries are emerging as a potential game-changer in energy storage technology. As we move toward more sustainable and eco-friendly energy solutions, salt-based batteries are gaining attention for their potential to replace the current dominance of lithium-ion (Li-ion) batteries. This article explores salt batteries, their advantages, the current stage of research, scope for improvements, and why they could outperform lithium-ion batteries.
What Are Salt Batteries?

Salt batteries are a type of electrochemical energy storage system that uses salts, often in the form of sodium (Na+), as the primary charge carrier. Unlike lithium-ion batteries that rely on lithium (Li+), salt batteries use more abundant and less expensive materials, making them an attractive alternative for large-scale energy storage applications.
Advantages of Salt Batteries
1. Abundant and Low-Cost Materials
One of the most significant advantages of salt batteries is the use of abundant materials. Sodium, which is commonly used in salt-based batteries, is far more abundant and cheaper than lithium. Sodium is widely available, which could dramatically reduce the cost of battery production and lead to more affordable energy storage solutions in the future.
2. Environmental Benefits
Salt-based batteries, especially those using sodium, are more environmentally friendly compared to lithium-ion batteries. The extraction of lithium is often associated with environmental degradation, including the destruction of habitats and pollution. In contrast, sodium is widely available and does not pose the same environmental risks.
3. Enhanced Safety
Salt batteries are generally safer than lithium-ion batteries. Lithium-ion batteries can overheat and catch fire under certain conditions, particularly when damaged or improperly charged. Salt-based batteries, however, tend to be less prone to overheating or thermal runaway, making them safer for use in various applications, from electric vehicles (EVs) to grid storage.
4. Longer Lifespan
Salt batteries are believed to have the potential for a longer lifespan compared to lithium-ion batteries. This is due to the lower risk of degradation and capacity loss over time. Salt-based batteries are expected to retain their efficiency and performance for more charge-discharge cycles, making them a better option for long-term storage solutions.
5. Higher Efficiency at Low Temperatures
One of the challenges faced by lithium-ion batteries is their efficiency drop at low temperatures. Salt batteries, particularly sodium-ion batteries, tend to perform better in cold conditions, making them more suitable for use in regions with colder climates or for applications requiring stable performance in extreme conditions.
Current Stage of Research
Salt batteries, particularly sodium-ion batteries, are still in the research and development phase. While they are not yet as commercially viable as lithium-ion batteries, several breakthroughs have been made in recent years:
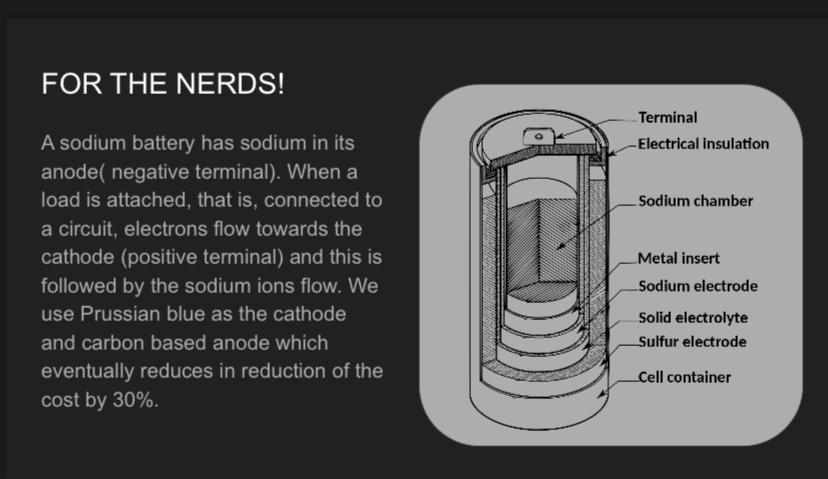
1. Improved Performance
Researchers have been working on improving the performance of salt-based batteries by enhancing their energy density and charging speed. In early models, sodium-ion batteries were less efficient than lithium-ion batteries. However, recent advancements have resulted in prototypes that show comparable energy densities, making them more competitive.
2. Better Electrolytes and Anode Materials
The development of advanced electrolytes and anode materials is a key area of focus. Sodium-based electrolytes and better anode materials have been developed, improving the efficiency, lifespan, and overall performance of salt batteries. Researchers are experimenting with materials such as hard carbon and Prussian blue analogs to boost the performance of these batteries.
3. Commercial Pilots
Several companies and research institutions are currently working on pilot projects to scale up the production of salt-based batteries. While the technology is not yet mass-produced, small-scale prototypes and trials are being conducted, showing promise for future applications in energy storage systems.
Scope for Further Improvements
Despite the promising advancements, there is still significant room for improvement in salt battery technology. Some of the key areas where research can focus include:
1. Energy Density
One of the main challenges for salt batteries is improving their energy density. While sodium-ion batteries are more abundant and cost-effective, they generally have lower energy density compared to lithium-ion batteries. To compete with lithium-ion batteries in applications like electric vehicles, salt-based batteries must reach higher energy densities.
2. Cycle Life
The long-term durability of salt batteries, although promising, still requires improvement. Salt batteries need to undergo more rigorous testing to ensure they can withstand many charge-discharge cycles without significant performance degradation. Advancing electrolyte formulations and optimizing the anode/cathode structures can help extend the cycle life of salt batteries.
3. Charging Speed
Salt batteries generally take longer to charge than lithium-ion batteries. This is another area of active research, with efforts to improve the ionic conductivity of the electrolyte and reduce internal resistance, which will ultimately lead to faster charging times.
4. Scalability and Manufacturing
For salt-based batteries to become commercially viable, the production process needs to be scaled up. This involves overcoming challenges in manufacturing and ensuring that the raw materials required for salt batteries are readily available at a large scale. Developing cost-effective, mass-production techniques is essential for making these batteries accessible for widespread use.
How Salt Batteries are Better Than Lithium-Ion Batteries

1. Cost-Effectiveness
Sodium is abundant and much cheaper than lithium, which can make salt batteries more cost-effective in the long run. As the demand for energy storage systems grows, the affordability of salt-based batteries could play a significant role in driving their adoption for large-scale energy storage.
2. Sustainability
Salt batteries offer a more sustainable solution for energy storage. The mining of lithium has raised concerns due to its environmental impact. Sodium, on the other hand, is abundant, non-toxic, and easier to extract, making salt batteries a more eco-friendly alternative.
3. Better Performance in Extreme Conditions
Salt batteries are more resilient in extreme temperatures, especially in colder climates. In contrast, lithium-ion batteries tend to lose performance in freezing conditions. This makes salt-based batteries a more versatile option for various industries, including transportation and grid storage, where temperature control is a factor.
4. Safety
Lithium-ion batteries have a known risk of overheating, fire, and explosions, particularly when subjected to physical stress. Salt batteries, however, are less likely to overheat and are generally more stable. This makes them safer for both consumer use and large-scale industrial applications.
Conclusion
Salt batteries, particularly sodium-ion batteries, have the potential to revolutionize energy storage technologies by providing a safer, more sustainable, and cost-effective alternative to lithium-ion batteries. While they are still in the research phase, significant advancements are being made in terms of energy density, cycle life, and charging speed. With further improvements and mass production, salt batteries could become a key player in the future of energy storage, driving the transition to a greener, more affordable energy future.